High concentration of silicon. You know how
Processor? Pisok? And what do you have with the word associations? Or maybe Silicon Valley?
As if it wasn’t there, with silicon we’re sticking today, and if you need to know, what is Si and why do you eat, I ask for a cat.
Entry
Being a student of one of the Moscow universities in the specialty "Nanomaterials", I want to know you, dear reader, with the most important chemical elements of our planet. I’ve been choosing something for a long time, charcoal or silicon, and yet I’ve decided to use Si myself, so that the heart of any modern gadget is based on it itself, so you can say so splendidly. I’ll try to make my thoughts extremely simple and accessible, having written this material, I’ve saved it, mainly on newbies, but more and more people can get it from cicave, so I would like to say that the article was written exclusively for expanding interests, what got stuck. And so let's get started.
Silicium
Silicon (lat. Silicium), Si, chemical element of the IV group of the Mendeliev periodic system; atomic number 14, atomic weight 28.086.
In nature, the element is represented by three stable isotopes: 28Si (92.27%), 29Si (4.68%) and 30Si (3.05%).
Thickness (n.s.) 2.33 g/cm?
Melting point 1688 K

Powder Si
Historical proof
Hills of Silicon, wide lands, boulevards of people from a stone age. There were thousands of sprats of stone and the watering of the stone. Zastosuvannya spoluk Silicon, tied with their processing, - the preparation of a warehouse - began around 3000 BC. e. (Old Egypt). Earlier in the house, silicon was used - oxide SiO2 (silica). In the 18th century, silica was injected with a simple body and carried to the "lands" (which is used in its name). Skladnіst to a warehouse of silica having installed І. I. Berzelius. Vіn same vpershe, in 1825, having removed elementary silicon from silicon fluoride SiF4, replacing the remaining metal potassium. The new element was named "silicium" (Lat. silex - flint). Russian name vvіv G. I. Hess at 1834 roci.

Silicon arc of extensions in nature at the warehouse of the sonic sound
Expanding silicon in nature
For the breadth in the earth's crust, Silicon is another (after sour) element, its average in the lithosphere is 29.5% (behind the mass). The earth's bark, Silicon gray, has the same primary role, like the charcoal of the creature's earthly light. For the geochemistry of Silicon, it is important, in particular, that the link between yogo and sour is important. Close to 12% of the lithosphere becomes silica SiO2 in the form of the mineral quartz and various varieties. 75% of the lithosphere is composed of various silicates and aluminosilicates (polova, mica, amphibole toshcho). The total number of minerals, which can be replaced by silica, exceeds 400.
Physical power of Silicon
I think it’s especially not varto, that’s all Physical power I have free access, but I will list the most common ones.
Boiling point 2600 °C
Silicon prozorium for dovgokhvilovyh ІCh-promenіv
Dielectric penetration 11.7
Mohs Silicon Hardness 7.0
I would like to say that the silicon tenditny material, commemorative plastic deformation begins at temperatures higher than 800°C.
Silicon is a conductor, the very same wine we know a lot of zastosuvannya. The electrical power of silicon can lie in the house.
Chemical power.
It’s rich here, it’s amazing, one might say, but I’m stumbling on the most famous one. In the shelves of Si (similar to carbon) 4-valent.
On the surface of the silicon zavdyak, the oxide smelting is stable to wind at elevated temperatures. In acid, it oxidizes at 400 °C, dissolving silicon oxide (IV) SiO2.
Silicon resistant to acids and is less likely to be mixed with nitric and hydrofluoric acids, easily distinguished in hot meadows from seeing water.
Silicon make 2 groups of acidic silanes - siloxane and siloxenium. Silicon reacts with nitrogen at temperatures above 1000 °C. it is a valuable material for the chemical industry, as well as for the production of fire extinguishers. High hardness, as well as thermal and chemical resistance, are treated with silicon carbide (silicon carbide SiC) and boron (SiB3, SiB6, SiB12) floors.
Otrimanya Silicon
I think tse naytsіkavіsha part, here is the report.
The fallow type of recognition is divided:
1.
Silicon electronic power(t.z. electronic silicon"") - the most silicious silicon out of the silicon mist over 99.999% behind the wall, the electrical support of the silicon electronic quality can be changed in the interval of approximately 0.001 to 150 ohm cm, but with which the value of the support is to blame buti is provided with an all-inclusive house, so that you can get into the crystal of others house, wanting to take care of the tasks of the nursery of the electric opir, ring out, unacceptable.
2.
Silicon sony quality(t.z. "sonyachny silicon") - silicon from mixed silicon over 99.99% per fiber, which is used for the production of photoelectric converting devices (sonic batteries).

3.
Technical silicon- blocks of silicon polycrystalline structure, taken by the method of carbothermic renewal from pure quartz squeak; cover with 98% silicon, the main house is coal, it is aired with a high amount of light elements - boron, phosphorus, aluminium; mainly vicariously used for the possession of polycrystalline silicon.
Silicon of technical purity (95-98%) possesses electric blasts of inspired silica SiO2 between graphite electrodes. At the link with the development of nap_v_dnikovoї tehnіko razrobleno method of obtaining pure and especially pure silicon. For the sake of the forward synthesis of pure vih_dnih spoluk silicon, z yakikh vityagyat silіkі vіdnovlennya vіdnovlennya аbo termіchnogo razkladannya.
Polycrystalline silicon ("polysilicon") - the most pure form of silicon, which is commercially viable, - a product that is used for the purification of technical silicon by chloride and fluoride methods and is victorious for the production of mono- and multi-crystalline silicon.
Traditionally, polycrystalline silicon is removed from technical silicon with a path of transferring yoga into taps of silane (monosilane, chlorosilane, fluorosilane) with an offensive bottom of silanes, which are settled, rectified purification of the converted silane and refurbishment of silane to metal silicon.
Pure silicon conductors are obtained in two types: polycrystalline(reinforcement of SiCl4 or SiHCl3 with zinc or water, thermal expansion of SiI4 and SiH4) and monocrystalline(Crucible-free zone melting and “winding” of a single crystal from molten silicon - the Czochralsky method).

Here you can use the Czochralski method to vibrate silicon.
Czochralski method- the method of vibrating the crystals with a path of winding up the hill in the air on the surface of the great obligation to melt with the initiation of the cob of crystallization with the path of bringing the seed crystal (or a large number of crystals) of a given structure and crystallographic orientation atsії in contact with a free surface melt.
Zastosuvannya Silicon
Specially alloyed silicon is widely used as a material for the preparation of electrical conductors (transistors, thermistors, power vibrators, thyristors; sony photocells that are used in spacecraft, as well as a lot of all sorts of things).
Shards of silicon prosoria for changing from long hairs in the size of 1 to 9 microns, it can be placed in infrared optics.
Silicon may be different and all areas of zastosuvannya, which are expanding. In metallurgy Si
vikoristovuetsya for the removal of sourness (deoxidation) from melted metals.
Silicon warehouse a large number of alloys of the hall and color metals.
Sound Silicon alloys to increase resistance to corrosion, improve their livar power and increase mechanical strength; prote with a greater zmistі Silicon can viklikati kryhkіst.
The most important are alloys, copper and aluminum alloys, which can be used to avenge belts.
Silica is processed by glass, cement, ceramic, electrical and other industrial galuzes.
Silicon cleaning is important for the manufacture of single electronic devices (for example, the processor of your computer) and single-chip microcircuits.
Pure silicon, used for pure silicon, purification of metallurgical silicon as crystalline silicon is the main raw material for sony energy.
Single-crystal silicon - the cream of electronics and sony power engineering is used for making mirrors of gas lasers.
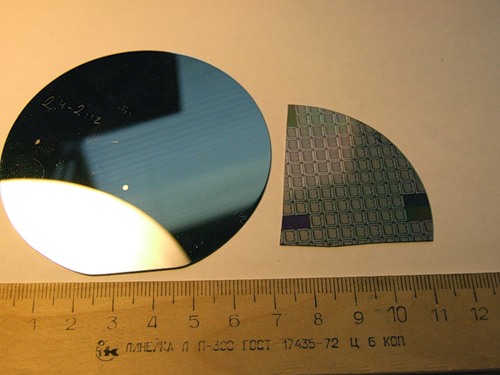
Cleaning silicon and a product of yoga
Silicon in the body
Silicon in the body is found in the eyes of various spoluks, who take part in the lead rank in the illumination of hard skeletal parts and tissues. Particularly rich in silicon can accumulate deaks of seaweed (for example, diatomaceous algae) and creatures (for example, silicon-horn sponges, radiolaria), which at the time of the ocean on the bottom of the ocean are hardened by deposits of silicon oxide (IV). In cold seas and lakes, biogenic mules, rich in silicon, are more important, in tropical seas - vaping mules with low content of silicon. Grasses, sedges, palm trees, and horsetails accumulate a lot of silicon in the middle of the terrestrial growths. In the backbone creatures, instead of silicon (IV) oxide, in the ash rivers 0.1-0.5%. In the largest number of silicon manifestations in the alkaline happy fabric, nirkah, pidshlunkovy vault. In a supplementary diet, people have up to 1 g of silicon. With a high intake of silicon (IV) oxide in the air, it is consumed in the legenia of a person that causes illness - silicosis.
Visnovok
Well, from everything, if you have read to the end and have penetrated a little, then you are a little closer to success. I spodіvayus, writing I not for nothing and pіst vouchsafed to hoch komu. For respect.
SILICON. (Silicium), Si - chem. element IV of the group of the periodic system of elements; at. n. 14, at. m. 28.086. Crystalline silicon-dark gray speech with a resinous sheen. In most cases, the degree of oxidation is revealed - 4, +2 and +4. Natural silicon is made up of stable isotopes 28Si (92.28%), 29Si (4.67%) and 30Si (3.05%). Withdrawal of radioactive isotopes 27Si, 31Si and 32Si with periods of reverse decay of 4.5 seconds, 2.62 years and 700 years. first seen in 1811 French. chemist and physicist J. L. Gay-Lussac and French. chemist L. J. Tenar, but there are fewer identifications in 1823 Swede, chemist and mineralogist J. Ya. Berzelius.
For the breadth in the earth's crust (27.6%), silicon-other (after sour) element. Knowing is important. in the form of silica Si02 and other sour speeches (silicates, aluminosilicates, etc.). For the best minds, a stable napkin modification is established, which has a face-centered cubic structure like diamond, with a period of a = 5.4307 A. Intermittent penetration is 2.35 A. Width is 2.328 gcm. With a high pressure (120-150 kbar) go to the larger wires and metal modifications. Metal modification-superconductor with a transition temperature of 6.7 K. With increasing pressure, the melting point decreases from 1415 ± 3 ° C at a pressure of 1 bar to 810 ° C at a pressure of 15,104 bar rare). ). When melted, there is an increase in the coordination number and metalization of the interatomic bonds. Amorphous silicic character of the short-range order, which shows a strongly created volume-centered cubic structure, close to rare. Debievskaya t-ra is close to 645 K. Coef. temperature linear expansion changes with changing t-ri according to the extreme law, lower than t-ri 100 To wine becomes negative, reaching the minimum (-0.77 10 -6) deg -1 at t-ri 80 K; at t-rі 310 K wine is 2.33 10-6 deg-1, and at t-rі 1273 K-4.8 10 deg-1. Heat of fusion 11.9 kcal/g-atom; tkіp.3520 K.
The heat of sublimation and evaporation at melting temperature is 110 and 98.1 kcal/g-atom. Thermal conductivity and electrical conductivity of silicon lie in the purity and thoroughness of crystals. Zі rostannyam t-ri coef. the thermal conductivity of pure K. gradually increases (up to 8.4 cal/cm X X sec deg at t-ri 35 K), and then changes, reaching 0.36 and 0.06 cal/cm sec deg at t-ri vіdpovidno 300 and 1200 K. Enthalpia, entropy and heat capacity K. in standard minds, it is equal to 770 cal/g-atom, 4.51 and 4.83 cal/g-atom - deg. Silicon diamagnetic, magnetic susceptibility of solid (-1.1 10 -7 mu/g) and rare (-0.8 10 -7 mu/g). Silicon is weakly deposited in t-ri. Surface energy, thickness and kinematic viscosity of rare K. at melting temperature become 737 erg/cm2, 2.55 g/cm3 and 3 10 m2/sec. A typical crystalline silicon conductor with a fenced zone width of 1.15 ev at t-p 0 to 1.08 u - at t-p 300 K. At room temperature, the concentration of wet-carrying charges is close to 1.4 10 10 cm - 3;
The power of silicon to fall due to the nature and concentration of houses, as well as to the perfection of the crystal. Ring for otrimannya napіvprovіdnikovogo Do. with the conduction of r- and n-type yoga, they are combined with elements IIIb (boron, aluminium, gallium) and Vb (phosphorus, mish'yak, antimony, bismuth) subgroup, which create sukupnіst vіdpovіdno to acceptor and donor rіvnі in, roztashovannyh near the cordons of zones. For the alloying of vicorist, those other elements (for example,) are shaped so. glybokі rivnі, yakі obumovlyuyut burying and recombination of nosesіїv charges. Tse allows you to take materials with high electro. support (1010 ohm cm at t-rі 80 K) that small trivality of the base of minor wear charges, which is important for the increase in the number of different outbuildings. Coefficient thermoelectric power with silicon is just to be deposited in the t-ri and in the place of the house, zbіlshyuyuchi zі zrostannym elektroopor (at p = 0.6 ohm - cm, a = 103 microvolts / hail). The dielectric penetration of silicon (vіd 11 to 15) is weakly deposited in the warehouse and the thoroughness of monocrystals. The laws of optical claying of silicon are greatly changed due to the change in purity, concentration, and the nature of defects in life, as well as longevity.
The cordon of indirect claying of electromagnetic kolivan is close to 1.09 euros, direct claying - up to 3.3 euros. In the visible region of the spectrum of the parameters of the complex indicator of fracture (n - ik), it is already possible to lie on the surface of the house. For especially pure Do. (atλ \u003d 5461 A and t-re 293 K) n \u003d 4.056 and k \u003d 0.028. The robot output of electronics is close to 48 euros. Silicon trend. Yogo hardness (t-ra 300 K) for Moos - 7; HB = 240; HV y \u003d 103; I = 1250 kgf/mm2; modulus of norms, elasticity (polycrystal) 10890 kgf/mm2. Between the minerality to lie down in the depth of the crystal: on the vigin, the depth is 7 to 14; coef. Stiffness 0.325 1066 cm2/kg.
At room temperature, silicon practically does not interact with gas-like (including) and solid reagents, krіm lіv. When moving t-ri, there is an active interaction with metals and non-metals. Zocrema, which dissolves SiC carbide (at temp. 1600 K), Si3N4 nitride (at temp. 1300 K), phosphide SiP (at temp. 1200 K) and arsenide Si As, SiAS2 (at temp. 1000 K). With sour reaction at a temperature of 700 K, quenching Si02 dioxide, with halogens - SiF4 fluoride (at a temperature of more than 300 K), SiCl4 chloride (at a temperature of more than 500 K), SiBr4 bromide (at a temperature of 700 K) i nodide SiI4 (at mp 1000). Intensively reacting with many others. metals, establishing solid differences in their substitution for chi chem. on the half - silicides. Concentration areas of homogeneity of solid roses occur depending on the nature of the rosette (for example, Nimechchin in 0 to 100%, in the fall up to 15%, in alpha zirconium less than 0.1%).
The difference between metals and non-metals in solid silicon is significantly less and sounds retrograde. With what borders, instead of a house, what is being done at Do. not very deep, reach the maximum ( 2 10 18 , 10 19 , 2 10 19 , 1021, 2 10 21 cm) area t-r vіd 1400 to 1600 K. Houses with deep rіvenny vіdznyayutsya slightly smaller rozchinnіstyu (vіd 1015 for selenium and 5 10 16 for zalіza up to 7 10 17 for nickel and 10 18 cm-3 for midi). At a rare steel, silicon is not surrounded by common metals, often with great visions of heat. Pure silicon is prepared from a technical product 99% Si і - 0.03% Fe, Al і Co), tempered with quartz and coal in electric furnaces. Start with a new breath to-there (sum_shy hydrochloric and sirchanoy, and then fluoride-water and sirchanoy) houses, after which the product (99.98%) is taken away, treated with chlorine. Syntheses are purified by distillation.
Silicon conductor is used in combination with SiCl4 chloride (or SiHCl3) in water or thermal expansion of SiH4 hydride. Residual purification and grinding of monocrystals is carried out with a crucibleless zone smooth method or by the Czochralsky method, taking into account especially pure gravy (in the house up to 1010-1013 cm-3) cf\u003e 10 3 ohm cm. iv at which to enter dosing of the number of necessary houses. This is how you prepare cylindrical creams with a diameter of 2-4 and a length of 3-10 cm. For special. purpose is to produce even more monocrystals. Technical silicon and especially yogo alloys from vicorous saline like acidic steels and alloys, as well as light additives. Particularly pure zrazki single-crystal K., doped with various elements, is known to be used as the basis of various low-current (zocrema, thermoelectric, radio, light-and phototechnical) and high-current (vibrating, converting) attachments.
Silicium and silicon
Silicon is brought to non-metals, and 4 electrons can be on the same energy level. Vіn can vіddavati їх, showing the oxidation stage + 4 and add electrons, showing the oxidation stage - 4. However, the ability to add electrons to silicon is significantly less, lower to carbon. Silicon atoms can have a larger radius, lower carbon atoms.
Nature's knowledge of silicon .
Silicon arch of extensions in nature. per її part of the fall pon over 26% of the mass earth measles. For the width of the wines, sit in another place (after sour). On the vіdmіnu vіd vіd uglec C vіlny stіnі vіrіdі not zustrichaetsya. Він enter to the warehouse of various chemical compositions, mainly various modifications of silicon oxide (IV) and salts of silicic acids (silicates).
Obsession with silicon .
Industrial grade silicon of technical purity (95 - 98%) is dominated by SiO 2 coke in electric ovens during roasting:
SiO 2 + 2C \u003d Si + 2CO
SiO 2 + 2Mg \u003d Si + 2MgO
In this way, amorphous silicon powder of brown color is taken from the houses. Recrystallization from melting metals (Zn, Al) can be transferred to a crystal mill.
Silicon tetrachloride, even of high purity, is used at 1000°C for silicon tetrachloride SiCl 4 pairs of zinc:
SiCl 4 + 2Zn \u003d Si + 2ZnCl 2
that cleansing yogo following special methods.
Physical Chemical power silicon.
Pure crystalline silicon - tenditny and hard, flabby slope. Like a diamond, it has a cubic crystal lattice with a covalent type of bond. Yogo melting point 1423 °C. For the greatest minds, a silicon inactive element, which only reacts with fluorine, but when heated, enters into various chemical reactions.
Yogo vikoristovuyut as a valuable material for napіvprovіdnikovіy tehnіtsі. Paired with other conductors of wine, it is distinguished by a significant resistance against dilute acids and a great electrical resistance up to 300 ° C. Technical silicon and ferrosilicon vicorist also in metallurgy for heat-resistant, acid-resistant and tool steels, chavuns and rich other alloys.
With silicon metals chemistries, Called silicides, when heated with magnesium, magnesium silicide is dissolved:
Si + 2Mg = Mg 2 Si
Silicides of metals behind the structure of that dominance predict carbides, so metal-like silicides, just like metal-like carbides, are distinguished by great hardness, high melting point, hot electrical conductivity.
When frying sumish pisku with coke in electric furnaces, silicon carbide and carbon are mixed with silicon carbide, or carborundum:
SiO2 + 3C = SiC + 2CO
Carborundum is a refractory, barless hard speech, a valuable abrasive and heat material. Carborundum, yak i, maє atomic crystalline grati. At the clean station there is an insulator, but in the presence of the house, it becomes a conductor.
Silicon yak i , dissolves two oxides: silicon oxide (II) SiO and silicon oxide (IV) SiO 2 . Silicon oxide (IV) is a hard refractory speech, widely widened in nature in the free country. Tse chemically stable speech, which interacts only with fluorine and gas-like fluorine in water or hydrofluoric acid:
SiO 2 + 2F 2 \u003d SiF 4 + O 2
SiO 2 + 4HF \u003d SiF 4 + 2H 2 O
Pointing directly to the reactions is explained by the fact that silicon can have a great sporidness to fluorine. In addition, silicon tetrafluoride is a flying speech.
At tekhnіtsі prozoriya SiO 2 vykorovuyut for the preparation of a stable refractory quartz slab, as a kindly misses the ultraviolet change, may have a great coefficient of expansion, which shows the significant changes in temperature. Amorphous modification of silicon oxide (II) tripoli maє great porosity. Yogo vikoristovuyut as a heat and sound insulator for vibrating dynamite (nose vibukhovo speech) and so on. Silicon (IV) oxide in a vibrating sound is one of the main everyday materials. It is vicorous in the production of fire and acid-resistant materials, steel, like a flux in metallurgy and so on.
According to the molecular formulas, chemical and physical power of carbon oxide (IV) and silicon oxide (IV) is easy to match, the power of these is similar in chemical warehouse z'ednan raznі. Why is it explained that silicon oxide (IV) is formed not just from SiO molecules 2 , such as s їх associates, in some of them the atoms of silicon are joined together by the atoms of sour. Silicon (IV) oxide (SiO 2 )n. Image її on the square:
Another representative of the elements of the head subgroup of the IV group (IVA group) of the Periodic system of D. I. Mendeliev - silicon Si.
In nature, silicon is another chemical element for the width of the acid. The earth's crust is more and more quarterly folded from yogo spoluk. The most wide-spread silicon is silicon oxide (IV) SiO 2, the other name is silica. In nature, wines are made with the mineral quartz (small 158), a rich variety of such - girsky crystal, and also the famous lilac form - amethyst, as well as agate, opal, jasper, chalcedony, carnelian, in the form of virobnі and intoxicating stones. Silicon (IV) oxide is also composed of a remarkable and quartz sand.

Mal. 158.
Quartz crystals interspersed in dolomite
Three different minerals based on silicon oxide (IV) (flint, chalcedony and other) first people prepared znaraddya pracі. The same flint, which is inconspicuous and not narrower than a stone, laying the cob of a stone vіtsі - vіtsі kremіnyh znaryad pratsі (Fig. 159). There are two reasons for this: the breadth and availability of flint, as well as the construction of buildings with how sharp the edges are.

Mal. 159.
Znaryaddya stone vіku
Another type of natural materials for silicon is silicate. Among them are the largest widths of aluminosilicates (it was understood that these silicates can replace the aluminum chemical element). Granite is added to aluminosilicates, see different clay, mica. Silicate, which does not avenge aluminium, for example, asbestos, which is used to fabricate fireworks.
Silicon oxide (IV) SiO 2 is necessary for the life of growers and creatures. The wines give the stems of roslin and zahisnymi veils of creatures (Fig. 160). Zavdyaki yomu queues, queues and horsetails stand mitzno, like bagnets, gostre leafing sedges cut, like knives, stubble on a sloping field, like a head, and stalks of cereals on the floor of a mitzna, which do not allow cornfields in the fields to lie in a plank and wind . Luska rib, shells of coma, krill meteliks, pir'ya ptahiv і wool of creatures of mіtsnі, oskіlki smite silica.

Mal. 160.
Silicon (IV) oxide gives minerality to the stems of roslin and to the suffocating curves of creatures
Z'ednannya silicon gives smoothness and softness to the hair and nails of a person.
Silicon to enter and to the warehouse of the lower living organisms - diatoms and radiolarians, the lowest breasts of living matter, as if creating their imperfections behind the beauty of the skeleton with silica (Fig. 161).
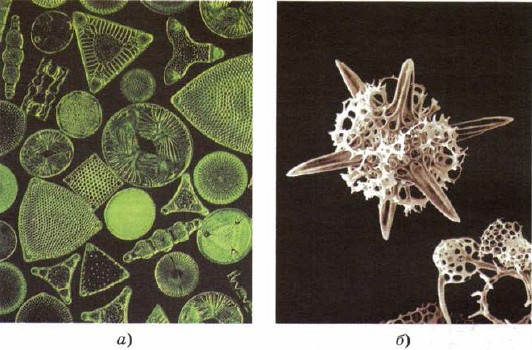
Mal. 161.
Skeletons of diatoms (a) and radiolarians (b) are composed of silica
The dominance of silicon. You are using a microcalculator with a sony battery, and so there is a notice about crystalline silicon. Tse napіvprovіdnik. On the vіdmіnu vіd metalіv, іz pіdvishennyam temperature yogo elektroprovіdnіst zbіshuєtsya. On satellites, spaceships, stations and dahah budinkiv (small 162) they install sony batteries that convert sony energy into electricity. The stench works crystals of napіvprovіdnikіv, and at the first black flint. Silicon photocells can be converted to electricity up to 10% of clayed sony energy.

Mal. 162.
Sleepy battery for dahu booth
Silicon to burn at the acidity, satisfying you with silicon oxide (IV):
![]()
Being non-metal, when heated, silicon merges with metals with silicide solutions, for example:
![]()
Silicides are easily spread with water or acids, when you see a gas-like water-like silicon - silane:
On the surface of carbohydrates, silane on the surface self-engages and burns with dissolved silicon oxide (IV) and water:
The increased reaction between silane and methane CH 4 is explained by the fact that the expansion of the silicon atom is larger, lower at the carbon, so the Si-H chemical bonds are smaller, the C-H bonds are lower.
Silicon interplay with concentrated water changes of meadows, making silicate and water:
Silicon possess, similar to silicon oxide (IV) magnesium or carbon:
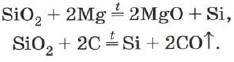
Silicon oxide (IV), or silicon dioxide, or silica SiO 2 yak і CO 2 є acidic oxide. However, on the surface of CO2, there is a molecular, but an atomic, crystal lattice. To that SiO 2 is hard and refractory speech. Wines do not differ in water acids, hydrofluoric cream, and aloe at high temperatures with meadows with dissolved salts of silicic acid - silicates:
Silica can also be used to alloy silicon oxide (IV) with metal oxides or carbonates:
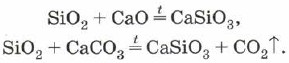
Sodium silicates and potassium are called retail slabs. Їх water supply- It's good to use silicate glue.
Silic acid H 2 SiO 3 (Fig. 163):
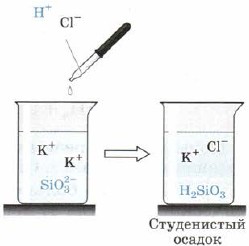
Mal. 163. Acid reaction to silicate-ion
Also, H2SiO3 is also a weak acid. It’s inconsistent in the water and falls out of the reactionary turmoil in looking at the dregs of the siege, which sometimes compresses the entire volume of the difference, transforming it into a solid mass, I go to the jelly, jelly. When hanging masi, a highly porous speech - silica gel is established, which is widely used as an adsorbent - clay of other speeches.
Laboratory Certificate No. 40
Possession of silicic acid and that vindication of power
Silicon injection. You already know that silicon is used for the production of conductive materials, as well as acid-resistant alloys. When quartz sand is fused with wool at high temperatures, silicon carbide SiC is dissolved, which is only available to diamond. Therefore, it is necessary to vicorate for sharpening rіztsіv metalorіzalnyh verstatіv and polishing expensive stone.
From melted quartz, various chemical quartz dishes are prepared, which can withstand high temperatures and do not crack when cold.
Z'ednannya silicon є the basis for the warping of the warehouse to cement.
Zvichayne error may warehouse, which can be expressed by the formula Na 2 O CaO 6SiO 2. Yogo is won at special stoving ovens with fused sumish soda, vapnyak and piska.
Vіdmіnna rice skla - zdatnіst rozm'yakshuvatisya і in the molten steel swell whether it's a form, as it is saved when the warehouse is caught. On the basis of which the utensils are made and the other wares are made.
Dodatkovі yakosti sklu give different additives. So, the introduction of lead oxide is reduced to a crystal-clear color, chromium oxide becomes green-colored, cobalt oxide is blue, etc. (Fig. 164).

Mal. 164.
Products from color warehouse
Sklo is one of the oldest winemakers of the people. Already 3-4 yew. For this reason, the war broke out in Egypt, Syria, Finiki and the Black Sea.
Sklo - the same material is not less than remіsnikіv, but also artists. The maister reached the highest level of perfection Ancient Rome, as if they were able to take away the color slope and work of their mosaics.

Mal. 165.
Colored glass at the stained glass windows of Notre Dame Cathedral, Chartres
Create works of art from the warehouse with obov'yazkovymi attributes of a great museum, and colorful stained-glass windows of the church, mosaic panels - bright butts of this (Fig. 165). In one of the premises of the St. Petersburg branch of the Russian Academy of Sciences, there is a mosaic portrait of Peter I, victorious M. V. Lomonosov (Fig. 166).

Mal. 166.
Mosaic portrait of Peter I
The areas of congestion are rather large. Tse vikonne, plyashkove, lampov, dzerkalne sklo; optical slope - from eyepieces to camera glasses; lenses of indistinguishable optical devices - from microscopes to telescopes.
The second most important material, coatings based on silicon, is cement. Yogo is used for baking clay and vape in special ovens that are wrapped.
If the powder of cement is mixed with water, then the cement is not firmly established, otherwise, as it is called alarm clocks, cement rozchin, which is step by step harder. When adding concrete to cement, or crushed stone, concrete should be taken off like a top coat. The materiality of the concrete is growing, so that a new frame is introduced into the new one - the cast concrete comes out, from which the walls of the panel, blocks of overlaps, fermi bridges, etc. are made.
The warehouse is engaged in the production of cement silicate industry. Vaughn also produces silicate ceramics - ceglu, porcelain (small 167), faience and wares from them.

Mal. 167.
Porcelain varieties
Vіdkrittya silicon. Even though in ancient times people were widely victorious in the use of silicon, silicon itself was first taken away in 1824 by the Swedish chemist J. Ya. Berzelius. However, 12 years before the new flint, J. Gay-Lussac and L. Tenard were taken away, ale vin duzhe zabrudneniy houses.
The Latin name silicum takes its cob from the Latin word silex-flint. The Russian name "silicon" resembles the walnut kremnos - "skel, skel".
New words that understand
- Natural sources of silicon: silica, quartz and other varieties, silica, aluminosilicate, asbestos.
- Biological significance of silicon.
- The dominance of silicon: napіvprovіdnikovі, interplay with sour, metals, meadows.
- Silane.
- Silicon (IV) oxide. Yoga Budova and power: interaction with meadows, basic oxides, carbonates and magnesium.
- Silicic acid and її salts. Rozchinne slo.
- Zastosuvannya silicon and yogo half.
- Sklo.
- Cement.
name the day
Most often in nature, silicon is found in a look like silica - a base based on silicon dioxide (IV) SiO 2 (about 12% of the earth's measles mass). The main minerals and rocks that are quenched by silicon dioxide are chain (rich and quartz), quartz and quartzite, flint, polov_spar. Silica and aluminosilicate are folded to another for the width in nature.
It is noted one by one the fact of the importance of pure silicon in its native appearance.
Otrimannya
Silicon will come out when frying crispy white pisku (silicon dioxide) with magnesium:
S i O 2 + 2 M g → 2 M g O + Si (\displaystyle ~(\mathsf (SiO_(2)+2Mg\ \rightarrow \ 2MgO+Si)))At what do you settle amorphous silicon , what can look like brown powder.
In industry, silicon technical purity is obtained by melting SiO 2 with coke at a temperature of about 1800 ° C in ore-thermal furnaces of the shaft type. The purity of silicon removed by such a grade can reach 99.9% (the main houses are coal, metal).
You can move away from the purification of silicon from the houses.
- Purification in laboratory washrooms can be carried out by a path of the frontal possession of magnesium silicide Mg 2 Si. We gave magnesium silicide for additional hydrochloric or octic acids to remove gas-like monosilane SiH 4 . Monosilane is purified by rectification, sorption and other methods, and then placed on silicon and water at a temperature of about 1000 °C.
- Purification of silicon on an industrial scale is carried out by a path of non-intermediate chlorination of silicon. With this, the half-folds of the warehouse SiCl 4, SiHCl 3 and SiH 2 Cl 2 are dissolved. Їх in a different way clean the house (as a rule, by distillation and disproportionation) and at the final stage add pure water at temperatures from 900 to 1100 °C.
- Cheaper, cleaner and more efficient industrial technologies for silicon purification are being developed. For 2010 before them it is possible to introduce technologies for the purification of silicon with vicarious fluorine (replacing chlorine); technologies that transfer distillation to silicon monoxide; technologies based on stained-glass houses that focus on inter-crystal boundaries.
Vmіst domіshok at the finalized silicon can be reduced to 10 -8 -10 -6% per mass. More reports on the nutrition of pure silicon are reviewed in the article Polycrystalline silicon.
The method of obtaining silicon in a pure form of fragmentation by Mikola, Mikolayovich Beketov.
Physical power
The silicon crystal lattice is cubic face-centered like diamond, parameter a = 0.54307 nm (at high vise remove other polymorphic modifications to silicon), but through a larger bond between Si-Si atoms in a bond with a bond zv'azku S-S the hardness of silicon is significantly less than that of diamond. Silicon crackling, only when heated at 800 ° C, it becomes plastic speech. Vіn prozory for infrachervonogo viprominyuvannya s dozhini khvili 1.1 microns. The wet concentration of the nose in the charge is 5.81 10 15 m-3 (for a temperature of 300 K).
Electrophysical power
Elementary silicon in a single-crystal form is an indirect-gap conductor. The width of the fenced area at room temperature storage 1.12 eV, and at T \u003d 0 K - 1.21 eV. The concentration of wet charge carriers in silicon for normal minds becomes close to 1.5·10 10 cm −3 .
On the electrotrophic power of crystalline silicon, a great influx builds houses that roam in the dark. To extract silicon crystals with a deep conductivity, introduce atoms of elements of group III into silicon, such as boron, aluminium, gallium, and indium. To extract crystals from silicon with electronic conductivity to silicon, introduce atoms elements V-ї groupi, such as phosphorus, mish'yak, surma.
When electronic devices are assembled on the basis of silicon, it is important to attach the surface ball to the material (up to tens of microns), so the surface quality of the crystal can be added to the electrostatic power of silicon and, apparently, on the power of the finished tool. In the course of the assembly of certain devices, vicorous is added, applied to the modification of the surface, for example, the surface is coated with silicon with various chemical agents and її oprominennya.
Chemical power
Similar to carbon atoms, silicon atoms are characterized by sp 3 -hybridization of orbitals. At the link with hybridization, pure crystalline silicon makes diamond-like grains, in which silicon is chotivalent. At the same time, the silicon sound also manifests itself as a chotivalent element with an oxidation state of +4 or -4. Zustrichayutsya bivalent half silicon, for example, silicon oxide (II) - SiO.
For normal minds, silicon is chemically inactive and actively reacts only with gas-like fluorine, with which volatile silicon tetrafluoride SiF4 is dissolved. Such “inactivity” of silicon is due to the pasivation of the surface of the nanosized ball of silicon dioxide, which nega- tively settles in the presence of sour, again and again water (water vapor).
acidification with the dissolved SiO 2 dioxide, the process is accompanied by an increase in the volume of the dioxide ball on the surface, the stability of the oxidation process is limited by the diffusion of atomic acid and cryopreservation dioxide.
When heated to a temperature of over 400-500 ° C, silicon reacts with chlorine, bromine and iodine - with the adoption of readily volatile tetrahalides SiHal 4 and, possibly, halides in a folded warehouse.
With water silicon without a middle does not react, but with water silicon - silanes with the formula Si n H 2n + 2 - possess an indirect way. Monosilane SiH 4 (yogo is often called simply silane) is seen in the interaction of metal silicides with acids, for example:
C a 2 S i + 4 H C l → 2 C a C l 2 + S i H 4 (\displaystyle ~(\mathsf (Ca_(2)Si+4HCl\ \rightarrow \ 2CaCl_(2)+SiH_(4)\ ) uparrow)))SiH 4 silane, which dissolves in this reaction, avenges houses and other silanes, zokrem, Si 2 H 6 disilane and Si 3 H 8 trisilane, in some lancets for silicon atoms, bound together by single bonds (- Si -Si-Si-) .
Silicon reacts with nitrogen and boron at temperatures close to 1000 ° C, dissolving nitride Si 3 N 4 and thermally and chemically stable boride SiB 3 , SiB 6 and SiB 12 .
At temperatures above 1000 ° C, it is possible to use silicon that is the closest analogue to the periodic table - carbon - silicon carbide SiC (carborundum), which is characterized by high hardness and low chemical activity. Carborundum is widely used as an abrasive material. With this, however, the melting of silicon (1415 °C) can be contacted for three hours with coal in view of the large pieces of sintered fine-grained graphite of isostatic pressing, practically not changing and not interfering with the rest.
The lower elements of the 4th group (Ge, Sn, Pb) are uncoated in silicon, as well as most other metals. When heated with silicon and metals, their half-shells, silicides, can be formed. Silicides can be subdivided into two groups: ionic-covalent (silicides of puddle, puddle-earth metals and magnesium type Ca 2 Si, Mg 2 Si and in) and metal-like (silicides of transitional metals). Silicides of active metals are laid out under the dilution of acids; Metal-like silicides have a high melting point (up to 2000 °C). Metal-like silicide warehouses are most often used Me Si, Me 3Si2, Me 2 Si 3 , Me 5 Si 3 i Me Si 2 . Metal-like silicides are chemically inert, resistant to sourness at high temperatures.
It is especially important to note that the silicon seals the eutectic sum, that allows the spiking (fusing) of materials for the sealing of ferrosilicon ceramics at temperatures that are noticeably lower, lower than the melting temperature of the seal and silicon.
When adding SiO 2 to silicon at temperatures above 1200 ° C, silicon oxide (II) - SiO is dissolved. This process is constantly supported by the production of crystals by silicon methods
Silicon at the forefront of visions in 1811. J.Gay-Lussac and L.Tenar when passing vapors of silicon fluoride over potassium metal, proteins are not described by them as an element. Swedish chemist J. Berzelius in 1823 giving a description of the silicon removed by him during the processing of potassium salt K 2 SiF 6 with metal potassium at high temperature. The new element was named "silicium" (Lat. silex - flint). The Russian name "silicon" was introduced in 1834 by the Russian chemist Herman Ivanovich Hess. At the translation of other Greek. krhmnoz- Kut, mountain.
Knowledge in nature, otrimannya:
In nature, silicon is found in vibrating dioxide and silicates in a different warehouse. Natural silicon dioxide is more important in the form of quartz, wanting to use other minerals - cristobalite, tridymite, whale, cousite. Amorphous silica accumulates in diatom deposits on the bottom of the seas and oceans - these deposits settled with SiO 2, entering the warehouse of diatoms and other ciliates.
Vilniy silicon can be used for roasting with magnesium crispy white pisku, which for a chemical warehouse can be cleaned with silicon oxide SiO 2 + 2Mg = 2MgO + Si. In industry, silicon technical purity is obtained by melting SiO 2 with coke at a temperature of about 1800 ° C in arc furnaces. The purity of silicon obtained by such a rank can reach 99.9% (the main houses are coal, metal).
Physical powers:
Amorphous silicon may look like a brown powder, the thickness of which is more dense 2.0 g / cm 3. Crystalline silicon - dark gray, gleaming crystalline speech, crystallized and hard, crystallizes in diamonds. This is a typical conductor (it is better to conduct electricity, lower insulator type rubber, and higher for a conductor - mid). Silicon crackling, only when heated at 800 ° C, it becomes plastic speech. Cіkavo, scho siliceous prosorium to infrachervony viprominyuvannya, beginning with a long wind 1.1 microns.
Chemical power:
Chemically silicon low-active. At room temperature, only reacts with gas-like fluorine, volatile silicon tetrafluoride SiF 4 dissolves at this temperature. When heated to a temperature of 400-500 ° C, silicon reacts with acid with dissolved dioxide, with chlorine, bromine and iodine - with dissolved readily volatile tetrahalides SiHal 4 . At temperatures close to 1000 ° C, silicon reacts with nitrogen to dissolve nitride Si 3 N 4 with boron - thermally and chemically stable boride SiB 3, SiB 6 and SiB 12. With aqueous silicon, it doesn’t react without a hitch.
For etching with silicon, the sum of hydrofluoric and nitric acids is most widely used.
Set up to the meadows.
Silicon is characterized by an oxidation step of +4 or -4.
The most important fields:
Silicon dioxide, SiO 2- (Silicon anhydride) ...
...
Silicic acids- weak, indistinct, utvoryuyuyutsya when adding acid to the size of the silicate looks like a gel (gelatin-like speech). H 4 SiO 4 (ortho-silicon) and H 2 SiO 3 (meta-silicon, or silicon) are only known to vary and irrevocably convert to SiO 2 when heated and dried. Solid porous product to enter - silica gel can be opened up on the surface and vicorous as an adsorbent of gases, a drying agent, a catalyst and a wear catalyst.
silicate- salts of silicic acids of the good (crym silicates of sodium and potassium) are not soluble in water. power.
Waterfalls- analogues of carbohydrates, silani, half-heartedly, in some atoms of silicon with a single bond, strength yakscho atom silicon z'єdnаnі podvіyny zv'yazkom. Lances and kiltsya are used similarly to carbohydrates. All powers can be self-employed, make vibes of sumish with repetition and easily react with water.
Zastosuvannya:
The most significant amount of silicon is known for the selection of alloys for the application of aluminum, copper and magnesium, and for the selection of ferosilicides, which may be important for the selection of steels and heating equipment. Silicon crystals zastosovuyut sony batteries and napіvprovіdnikovih outbuildings - transistors and diodes. Silicon also serve as syrovina for the production of organosilicon slabs, or siloxanes, staining with oil, oil, plastics and synthetic rubbers. Inorganic slugs silicon vicorist is used in the technology of ceramics and steel, as an insulating material and p'ezocrystals
For some organisms, silicon is an important biogenic element. Vіn enter to the warehouse of the supporting huts at the roslin and skeletal - at the creatures. Sea organisms – diatoms, algae, radiolaria, sponges – are concentrated in large areas of silicon. Great numbers horsetails and cereals concentrate with silicon, in the first black - Bamboo and Rice-like pidrodins, among them - planting rice. M'yazova tissue of a person to revenge (1-2) 10 -2% silicon, bone tissue - 17 10 -4%, blood - 3.9 mg / l. However, up to 1 g of silicon can be ingested into the body of a person today.
Antonov S.M., Tomilin K.G.
HF Tyumen State University, 571 group.
